Didactics
Organic Chemistry
Auditorium and laboratory exercises at first degree studies in Chemistry and Chemical Business.
 Chemical nomenclature, electron structure of organic compounds, atomic and molecular orbitals, hybridization, isomeric (constitutional, stereoisomeric). Alkanes, cycloalkanes, alkenes, alkines: preparation and reactivity. Radical substitution, addition to multiple bonds. Structure and stability of radicals and carbocations, regrouping of carbocations. Conjugated dienes, resonance. Electrophilic addition to alkines. Stereochemistry: chiral centers, enantiomers, diastereoisomers, meso compounds, racemic mixtures and their separation. Conformational analysis of cyclohexane. Aromatic compounds. Aromaticity criterion. Aromatic electrophilic substitution. Isomer of multi-substituted aromatic compounds. Mechanism of nucleophilic substitution of aromatic compounds. Alcohols, phenols, ethers and epoxides, synthesis and reactivity. Reactions with alkyl halides, dehydration, reactions with metals, oxidation, acylation. Nucleophilic substitution: SN1 and SN2. Elimination reactions: E1 and E2 - mechanism and stereochemistry. Aldehydes and ketones. Structure and properties of the carbonyl group. Nucleophilic addition of water, alcohols, amines and Grignard compounds to the carbonyl group. Aldol condensation, Cannizzaro reaction, Wittig reaction. Carboxylic acids and their derivatives. Synthesis of carboxylic acids and their reactivity. Esterification reactions, formation of acid halides, anhydrides, amides, etc. Substitution in the acyl group. Keto-enol tautomeria. Use of ethyl acetylacetate and diethyl malate in organic synthesis. Condensation reactions e.g.: aldol, Claisen. Amines, alkalinity and nucleophilicity. Synthesis and reactions of amines. Heterocyclic compounds. Construction and nomenclature. Reactions with electrophilic and nucleophilic reagents, oxidation and reduction, acid-base properties. Laboratory classes include basic laboratory techniques (thin-layer chromatography, TLC, crystallization, extraction) and synthesis of organic compounds (liquid and crystalline).
Chemical nomenclature, electron structure of organic compounds, atomic and molecular orbitals, hybridization, isomeric (constitutional, stereoisomeric). Alkanes, cycloalkanes, alkenes, alkines: preparation and reactivity. Radical substitution, addition to multiple bonds. Structure and stability of radicals and carbocations, regrouping of carbocations. Conjugated dienes, resonance. Electrophilic addition to alkines. Stereochemistry: chiral centers, enantiomers, diastereoisomers, meso compounds, racemic mixtures and their separation. Conformational analysis of cyclohexane. Aromatic compounds. Aromaticity criterion. Aromatic electrophilic substitution. Isomer of multi-substituted aromatic compounds. Mechanism of nucleophilic substitution of aromatic compounds. Alcohols, phenols, ethers and epoxides, synthesis and reactivity. Reactions with alkyl halides, dehydration, reactions with metals, oxidation, acylation. Nucleophilic substitution: SN1 and SN2. Elimination reactions: E1 and E2 - mechanism and stereochemistry. Aldehydes and ketones. Structure and properties of the carbonyl group. Nucleophilic addition of water, alcohols, amines and Grignard compounds to the carbonyl group. Aldol condensation, Cannizzaro reaction, Wittig reaction. Carboxylic acids and their derivatives. Synthesis of carboxylic acids and their reactivity. Esterification reactions, formation of acid halides, anhydrides, amides, etc. Substitution in the acyl group. Keto-enol tautomeria. Use of ethyl acetylacetate and diethyl malate in organic synthesis. Condensation reactions e.g.: aldol, Claisen. Amines, alkalinity and nucleophilicity. Synthesis and reactions of amines. Heterocyclic compounds. Construction and nomenclature. Reactions with electrophilic and nucleophilic reagents, oxidation and reduction, acid-base properties. Laboratory classes include basic laboratory techniques (thin-layer chromatography, TLC, crystallization, extraction) and synthesis of organic compounds (liquid and crystalline).
Spectrochemistry
Auditorium exercises at the first degree studies in the fields of Chemistry and Chemical Business and lecture and laboratory exercises at the second degree studies in the field of Chemistry.
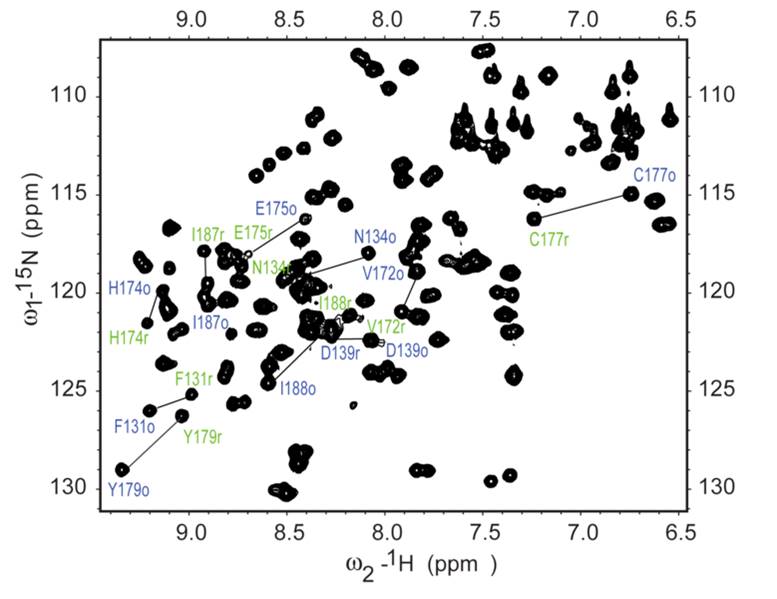 Theoretical and practical introduction to basic techniques: a) mass spectrometry (MS); b) electron and oscillatory absorptiometry (IR); and c) nuclear magnetic resonance spectroscopy (NMR). MS: electron ionization and chemical ionization (respectively, EI and CI), apparatus, types of decays and rearrangements in organic molecules, characterization of fragmentation ions, isotopes, exemplary MS spectra of organic compounds. IR: electromagnetic radiation, spectroscopic spectrum and molecular effect, harmonic and anharmonic oscillator, selection rules, types and classification of vibrations in chemical molecules, IR apparatus, formation of IR spectra – Fourier transformation, analysis of simple IR spectra of organic compounds; NMR: physical basis of 1H and 13C nuclei resonance (I = 1⁄2), FID signal acquisition-Fourier transform, chemical shift, coupling constant, 1H and 13C NMR spectrum and structural information contained therein, apparatus, analysis of NMR spectra of simple organic compounds, basis of multidimensional NMR spectroscopy.
Theoretical and practical introduction to basic techniques: a) mass spectrometry (MS); b) electron and oscillatory absorptiometry (IR); and c) nuclear magnetic resonance spectroscopy (NMR). MS: electron ionization and chemical ionization (respectively, EI and CI), apparatus, types of decays and rearrangements in organic molecules, characterization of fragmentation ions, isotopes, exemplary MS spectra of organic compounds. IR: electromagnetic radiation, spectroscopic spectrum and molecular effect, harmonic and anharmonic oscillator, selection rules, types and classification of vibrations in chemical molecules, IR apparatus, formation of IR spectra – Fourier transformation, analysis of simple IR spectra of organic compounds; NMR: physical basis of 1H and 13C nuclei resonance (I = 1⁄2), FID signal acquisition-Fourier transform, chemical shift, coupling constant, 1H and 13C NMR spectrum and structural information contained therein, apparatus, analysis of NMR spectra of simple organic compounds, basis of multidimensional NMR spectroscopy.




