Synthesis and properties of diosgenyl D-glycosaminosides
Saponins are structurally diverse group of glycosides widely distributed in nature, mainly in the plant kingdom. In the form of herbal extracts, ointments, various types of infusions, they are used there as antimalaria drugs, antidotes against venom of snakes and insects, and as antiseptics, bactericides and antivirus agents. The hydrophilic fragment of saponins is usually composed of one or more sugar units. The most common saccharides are D-glucose and L-rhamnose. Aglycon contains very often diosgenin.
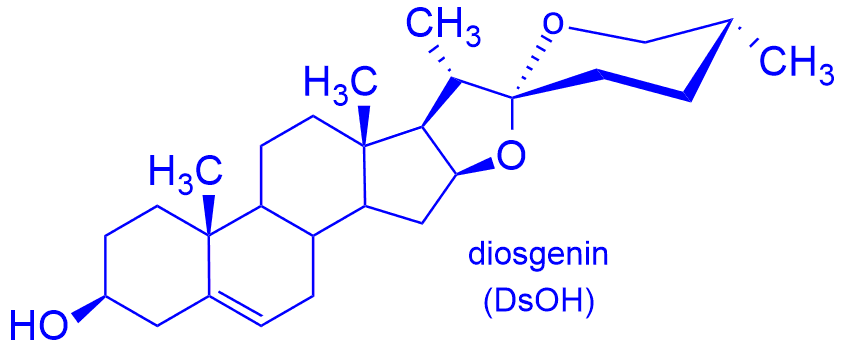
We have obtained a series of D-glycosides of diosgenyl containing the residue of D-glucosamine or D-galactosamine as sugar fragments. Such saponins are not found in nature. Their synthesis consists in skillfully combining the protected amino sugar with the glycosyl acceptor, i.e. diosgenin. The next step was the removing of the acetyl groups and the protecting group from the amino function, and finally to functionalize the amine group.[i-iv] We have obtained several derivatives, including hydrochloride 1, NH-acetyl derivatives 2, with the urea group 3, several N-alkyl 4 and N,N-dialkyl 5 – glycosides and also N-acyl derivatives containing residues of amino acids, including D- and L-alanine, sarcosine, L-serine, L-threonine or L-proline (Fig. 1).
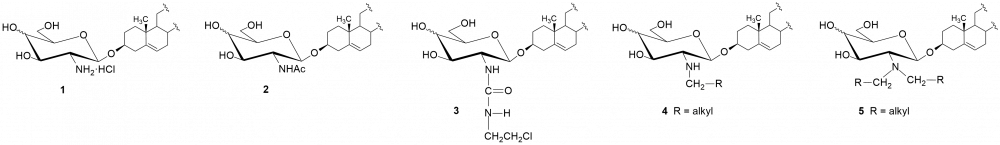
The obtained saponins have been tested to varying degrees for their antineoplastic and/or antimicrobial activity (bacteria and fungi).[v,vi]
[i] H. Myszka, D. Bednarczyk, M. Najder, W. Kaca, Carbohydr. Res., 2003, 338, 133-141. https://doi.org/10.1016/S0008-6215(02)00407-X
[ii] D. Bednarczyk, A. Walczewska, D. Grzywacz, A. Sikorski, B. Liberek, H. Myszka, Carbohydr. Res., 2013, 367, 10-17. http://dx.doi.org/10.1016/j.carres.2012.11.020
[iii] A. Walczewska, D. Grzywacz, D. Bednarczyk, M. Dawgul, A. Nowacki, W. Kamysz, B. Liberek, H. Myszka, Beilstein J. Org. Chem., 2015, 11, 869-874. https://doi.org/10.3762/bjoc.11.97
[iv] H. Myszka, P. Sokołowska, A. Cieślińska, A. Nowacki, M. Jaśkiewicz, W. Kamysz, B. Liberek, Beilstein J. Org. Chem., 2017, 13, 2310-2315. https://doi.org/10.3762%2Fbjoc.13.227
[v] O. Cirioni, H. Myszka, M. Dawgul, R. Ghiselli, F. Orlando, C. Silvestri, L. Brescini, W. Kamysz, M. Guerrieri, A. Giacometti, J. Med. Microbiol., 2011, 60, 1337-1343.
https://doi.org/10.1099/jmm.0.031708-0
[vi] D. Grzywacz, M. Paduszyńska, M. Norkowska, W. Kamysz, H. Myszka, B. Liberek, Bioorg. Med. Chem., 2019, 27, 114923. https://doi.org/10.1016/j.bmc.2019.05.036
| Attachment | Size |
|---|---|
| Diosgenina | 584.59 KB |




